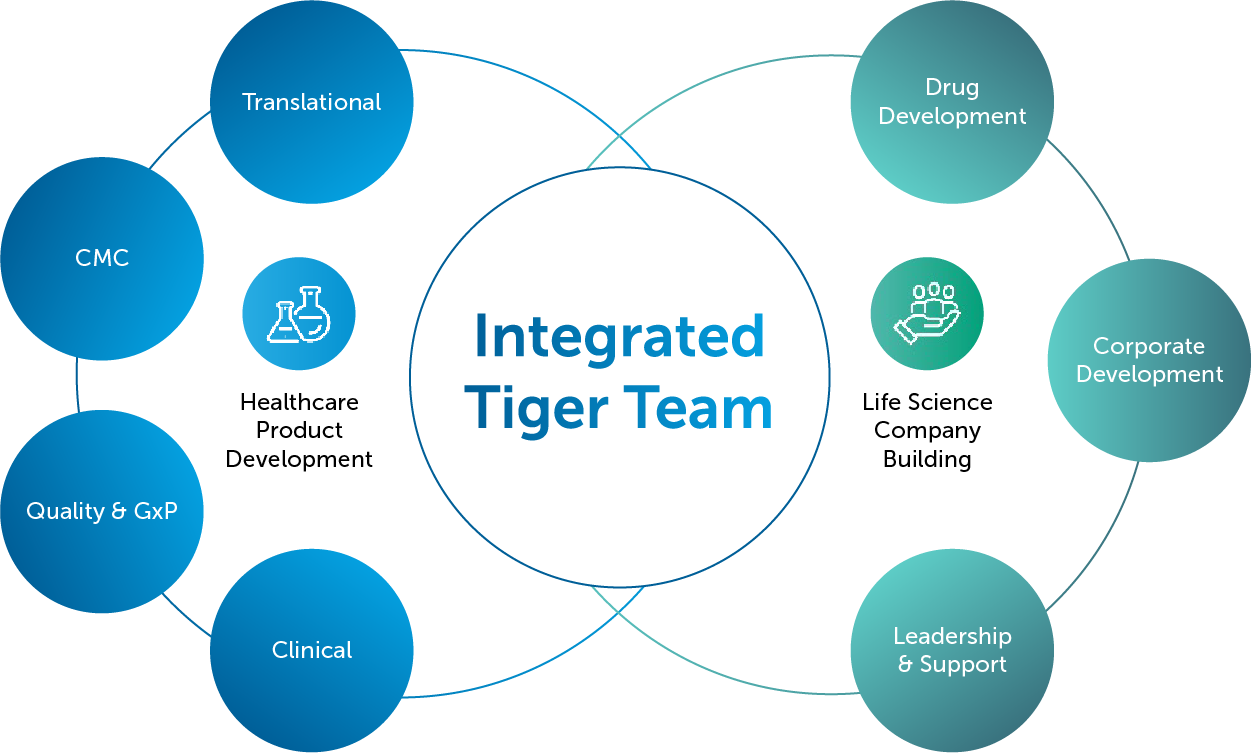
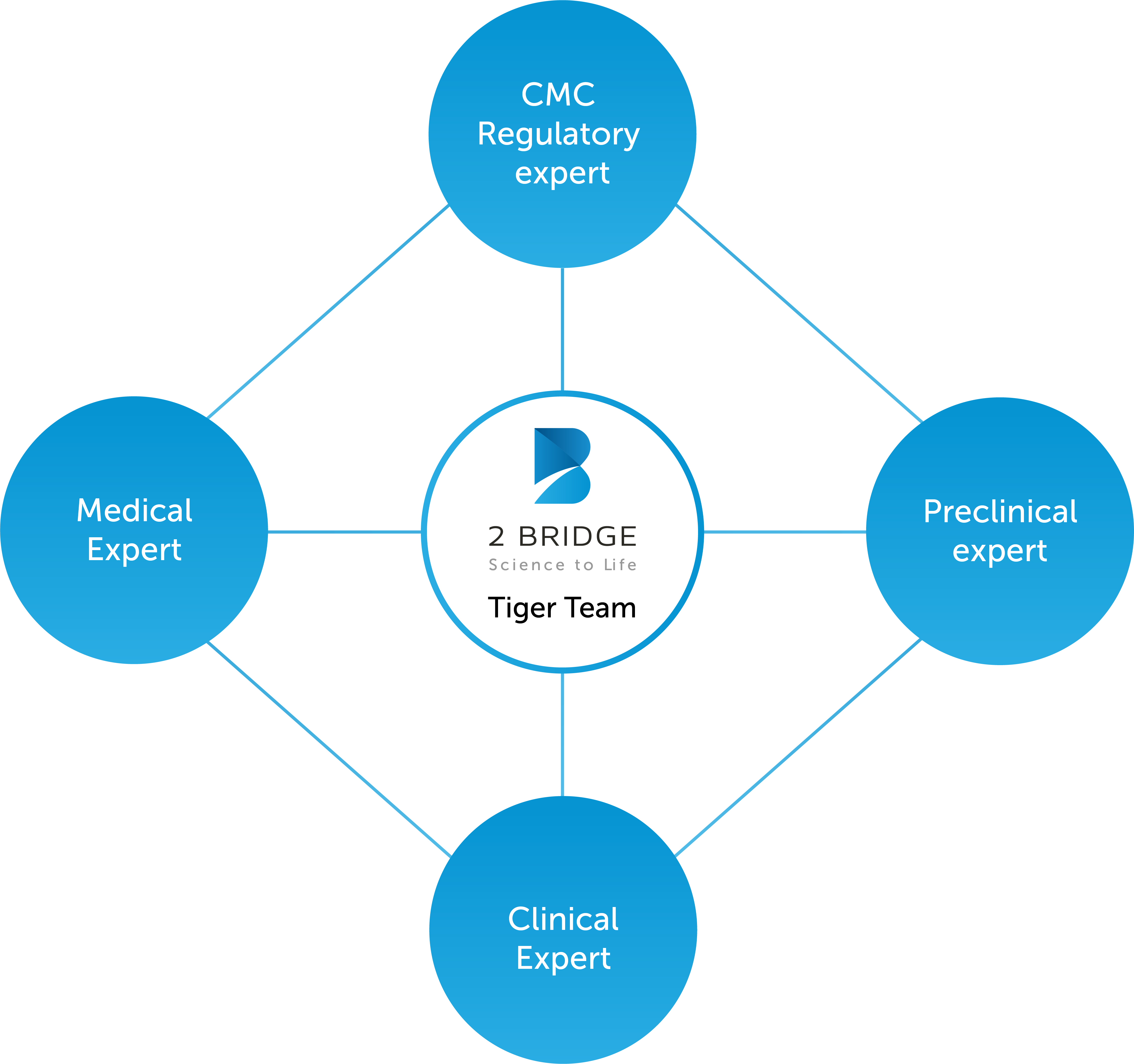
We take care of our clients’ projects as if they are our own by carefully assembling the 2 Bridge Tiger Team, a team of R&D experts and healthcare executives from various domains that provides knowledge, experience, and hands-on support.
Our Tiger Teams are agile and highly adaptable throughout the collaboration; ensuring you have flexible access to the right expertise at the right time; avoiding a cost-intensive trial-and-error process.